Engineering Mars commercial rocket propellant production for the Big Falcon Rocket (part 2)by Steve Hoeser
|
| An ideal additional processing design approach to produce the needed BFR oxidizer load would be one that avoids the production of the excess hydrogen. |
Once we have a supply of liquid purified water it can be broken down into hydrogen and oxygen. This can be accomplished using a process called electrolysis. The resulting hydrogen will feed the makeup hydrogen needed to produce methane gas needed in the Sabatier reactor (covered in the previous article) to complete the production of liquid methane fuel. The oxygen is needed to produce the oxidizer to burn the methane in the BFR rocket engines.
The water electrolysis processing is highly power intensive because of the energy of the bond strength between the hydrogen and oxygen atoms. The electrolysis of water requires a minimum of 237.13 kilojoules of electrical energy input to dissociate each mole of liquid water. Each mole of water gives you 2 grams of hydrogen and 16 grams of oxygen gases. Put another way, commercially available electrolysis systems require about 50 kilowatt-hours of power to produce one kilogram of hydrogen and eight kilograms of oxygen gas from nine kilograms of liquid water.
2H2O (g) ⇋ 2H2O (l)
2H2O (l) ⇋ 2H2 (g) + O2 (g)
Assuming that the final design determination is that electrolysis is the most cost-effective way to produce the oxygen (in gaseous form), the design specifications are the necessary first steps in creating this system. To get a full load of oxidizer for a SpaceX BFR, the electrolysis processor will need to produce 860 tonnes of oxygen for a full load (it is assumed that tons presented in the SpaceX data are metric tons, or 860,000 kilograms) of oxidizer. Using the molecular weight ratios, this means that around 986 tonnes of water will be needed to produce the needed BFR oxidizer load. This, of course, assumes that a full propellant load is required to lift any cargo and the BFR mass for its return trip to Earth.
For purposes of a first order gross review, system processing and BFR fueling losses are ignored or assumed otherwise compensated. For example, losses here on Earth for things like BFR tank pre-chills might be accomplished using alternative methods. This includes the use of excess nitrogen liquefied from the atmospheric separation process to do the majority of the pre-chill. A recirculation feed system designed to capture and reprocess liquefied oxygen may also be employed as part of the propellant loading processing design. Based on these assumptions, it requires almost 5.4 million kilowatt-hours, or just over 6 gigawatt hours of power, per BFR to produce the 860,000 kilograms of oxygen gas for a single fueling load.
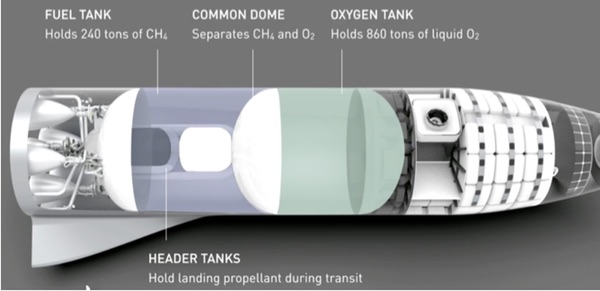 A fully loaded SpaceX BFR spaceship requires more than 1,000 tons of propellant. (credit: SpaceX) |
A propellant processing plant only using the Sabatier process makes the same amount of methane as oxygen. This means that additional processing capability is required to produce the three times more oxygen needed to obtain the proper combustion mix ratio of approximately four parts of oxygen to one part methane for use in the BFR Raptor engines. Since the Sabatier processing to produce the needed methane load produces around 539 metric tons of water, an additional 428 metric tons of water are needed to obtain the proper oxidizer BFR propellant load.
If water can be found and extracted on Mars or can be shipped to Mars cost effectively, the needed 428 metric tons of water can produce the needed additional oxygen using the electrolysis subsystems in the plant described earlier. This being the case, additional propellant production system processing design complexity would not be needed.
However, a good deal of extra hydrogen would result from the electrolysis of the extra water. If not dumped as waste to the Martian atmosphere, additional storage would be needed to house the excess hydrogen that is not used as input in the Sabatier production of the needed methane fuel. If system engineering design trades show that processing methods of waste dumping or extra storage tank systems are not found cost effective, other secondary processing system design options will be needed to use up the extra hydrogen.
2. An oxygen production alternative using solid oxide electrolysis of carbon dioxide: An ideal additional processing design approach to produce the needed BFR oxidizer load would be one that avoids the production of the excess hydrogen. One such method that has been suggested is to produce oxygen directly from atmospheric carbon dioxide using Solid Oxide Electrolysis (SOE), also called “zirconia electrolysis.” The SOE process involves the direct dissociation of carbon dioxide (which, as previously indicated, comprises 95 percent of the Martian atmosphere) into carbon monoxide and oxygen gas. This process produces one oxygen gas molecule out of every two carbon dioxide molecules processed. The carbon monoxide produced would either be a waste or could be retained, if it is determined to be a marketable byproduct.
| There are a number of fundamental disadvantages to the zirconia electrolysis system that have prevented it from being generally accepted as commercially practical for off-world applications. |
Conceptually, the zirconia electrolysis process is quite simple. Carbon dioxide gas is heated to temperatures of about 1000°C, where it partially dissociates into carbon monoxide and oxygen. The gas is run through thin-walled zirconia tubes, which are porous to oxygen transport. An electrochemical voltage potential is then set up between the inner and outer walls of the tube, causing the oxygen molecules to migrate across the tube. The pure oxygen gas can then be collected in the region surrounding the tube. The waste gas left inside the tube is a mixture of carbon dioxide (the majority) and carbon monoxide.1
There are a number of fundamental disadvantages to the zirconia electrolysis system that have prevented it from being generally accepted as commercially practical for off-world applications. One is that the oxygen output that can be generated by each of the tubes is quite small. This means that hundreds of the tubes would be required to produce propellant on the scale required.
Second, each of these tubes would have to be linked into a primary manifold in large single groups. This creates inherent points of failure in any design configuration. Specifically, the issue is that if a single tube within a group cracked or developed a leaky seal, the entire manifold group would be lost. Adding automated shut-off valves to each tube in the design to isolate faulty manifold groups adds design complexity, system costs and adds additional points of failure to the system.
Operationally, since the SOC tubes are made of brittle ceramic, and since high temperature/long duration seals are required for operation, such failure modes (and others) would be expected. Even worse, the required power to produce oxygen using these systems is unacceptably high when compared to other oxygen generation processes. By example, using a combined method such as the Sabatier and water electrolysis requires almost 9.5 times less energy and also is over three times as productive at producing oxygen.
3. Reverse water-gas shift (RWGS) reactors: Another proposed secondary design approach to produce the needed BFR oxidizer is to include an additional processing system that complements the Sabatier reactor processing. One such promising integrated Mars in situ propellant production system has been proposed by Robert M. Zubrin, Anthony C. Muscatello, and Mark Berggren in a paper published in the Journal of Aerospace Engineering, in January 2013.2 It includes a RWGS reactor compliment to the primary Sabatier process. This RWGS reactor uses additional carbon dioxide feed stock extracted from the Martian atmosphere and hydrogen gas to produce additional water and a carbon monoxide byproduct.
CO2 (g) + H2 (g) ⇋ CO (g) + H2O (g) ΔH = +41.2 kJ
The RWGS itself would consist of a simple compatible metal pipe, like steel, filled with a catalyst. According to experiments done by Pioneer Astronautics in Lakewood, Colorado, the best catalyst for this reaction is silica (consisting of five percent copper by weight) and a smaller amount of nickel. In the completed chemical process, one kilogram of carbon dioxide and hydrogen give you 0.3 kilograms of water. However, a RWGS reaction can go both ways, and this catalyst produces carbon monoxide with an efficiency of 60 percent conversion of carbon dioxide to carbon monoxide at 350°C under a pressure of 150 torr. This efficiency results because this reaction has a low equilibrium constant even at temperatures of 400°C. So to “push” the reaction toward production of the desired product, the RWGS reactor must be fed with either a hydrogen-rich or a carbon dioxide-rich mixture to ensure satisfactory results.
For each gram of carbon monoxide and water produced, the RWGS process takes a heat change equal to +41.2 kilojoules. This equates to the amount of heat consumed for the reaction of each molecule of carbon dioxide and water in the reactor. This produces water at a much lower total energy consumption than mining water.
| The current total power required for a BFR propellant load would be just over 12 gigawatts. |
The water vapor produced in the RWGS process would be fed into the already existing Sabatier water vapor liquefier in the plant. This additional water can then be stored in existing tanks. This volume of RWGS-generated water is fed into the electrolyzing processor to produce the needed oxygen propellant. The hydrogen created from the RWGS water in the electrolysis process is then set free. This means that, theoretically, all the hydrogen used in the RWGS can be recycled back into the RWGS reactor to react with more carbon dioxide from the atmosphere, creating a highly efficient cycle.
For purposes of this first order review, the initial electricity used to warm the RWGS reactor to initial operating temperatures is considered a previous “sunk” expenditure. Only the electricity needed to maintain the RWGS reactor operating temperatures with electric heaters is considered. Like the Sabatier reactor, the RWGS reactor will likely need to be extremely well insulated against the cold of Mars atmosphere.
Thoughtful engineers will likely point out that the additional maintenance reactor heat could also potentially be augmented during the day through the use of solar concentrator furnaces. If fission power reactors are available, these too might be designed to provide heat to the propellant production plant as well. Like all engineering, design trades will be used to determine the cost/value of such system design options.
Such reductions to Mars water mining and refining process created by the RWGS process will reduce the need for water mining and its associated power requirements. Combining the water need for the Sabatier process, the RWGS water and other processing system water recovery should reduce the mined water needs to only 162 metric tons. This correspondingly reduces the power need for water mining to just under 1.2 kilowatt-hours.
The decision to add the RWGS process to our propellant production plant means that the plant could produce the 698 metric tons of makeup water for the BFR oxidizer needed for a power expenditure of less than 0.2 kilowatt-hours.
4. Gases to Liquid Processing: Once the system produces the gaseous oxygen and methane, they must be liquefied before loading into the BFR. Before this liquefying processing can occur, though, any residual water must be removed from both gas streams. The drying subsystem used in this analysis is a modified process from Bock and Fisher’s study.3 It removes the moisture from the gaseous methane, hydrogen, and oxygen streams in a two-step process. In the first step, some 99.9 percent of the water is condensed in a cold trap separator. The second step removes the remaining moisture by absorption and adsorption via a corrugated rotor in the flow path, which is impregnated with a hygroscopic salt. The salt is regenerated by heated exit gas from the cold trap during a portion of the rotor revolution.
The actual “liquefaction” of gases requires another processing subsystem and more energy. Reasonable agreement exists in the collected data on oxygen liquefier performance. The correlations by Strobridge4 result in an estimate of 20 percent Carnot efficiency and 0.2 kilograms per watt for an oxygen liquefier in the capacity range of interest. Bock and Fisher5 and Kohout6 indicate 20 percent Carnot efficiency and 0.1 kilograms per watt, while Rocketdyne7 specifies a liquefier operating at 19 percent Carnot producing 0.4 kilograms per watt. Each process has its pros and cons so design trades prior to subsystem approach selection is needed.
For this first order review a 20 percent Carnot efficiency and 0.1 kilograms per watt (or 10 watts for every kilogram) produced is used to bring the oxygen gas to liquid temperature.8 So to liquefy 860 metric tons of oxygen gas for one BFR minimum energy Earth return mission requires just under ten megawatts. Combining this with the electrolysis energy in gas and then liquefying the oxidizer to just below boiling point requires about six gigawatt hours of power.
Like the oxygen, the methane gas will also need to be liquefied for use as rocket fuel. There are multiple methods for the liquefaction of the gaseous methane and each consumes different levels of power. This first order review of methane liquefaction processing is based on the electrical needs to produce one kilogram of liquefied natural gas (LNG) on Earth. The composition of LNG is mostly methane with small amounts of other hydrocarbons and nitrogen.
Quiang and co-authors9 considers that an amount of 850 kilowatt-hours per kilogram energy required to liquefy natural gas really corresponds to about 3 megajoules per kilogram. Gerasimov et al.10 proposed a plant in which the amount of energy consumed is about 700–800 kilowatt-hours per kilogram (2.5–2.8 megajoules per kilogram). In a textbook on natural gas, Medici believes a refrigeration cycle using a ternary mixture of refrigerants identified achieving a level of 1.9 megajoules per kilogram as a minimum for the energy required for compression of methane into liquid form.11
The current terrestrial technology that is one of the most energy efficient is an electric-drive liquefaction plant which uses 230 kilowatt-hours to make a tonne of LNG. Assuming an electric drive plant configuration, and the fact that this plant will not have to remove any of the hydrocarbon impurities or nitrogen found in terrestrial natural gas, a reasonably efficient Martian methane liquefaction processing plant appears possible.
| The observant reader will note that this seemingly large massive total BFR propellant production power estimated need is not required instantaneously. Instead, it will be spread out over an extended production run. |
Considering a pressure of the electric-drive liquefaction process of 55 bar (the critical pressure of methane is 46 bar) and a compression efficiency in the range between 0.8 and 0.85 for liquefying methane (plus overhead), electric requirements around 230 kilowatt-hours consumed per 1,000 kilograms of methane produced is reasonable. This puts the power needed to liquefy a BFR single full load of 240 metric tons of liquid methane at around 55.2 megawatts every 26 month optimal Earth/Mars launch position production run.
Energy Totals Check: Adding the major electrical energy needs for each step up to this point to produce storable near boiling point liquid oxygen and liquid methane, the current total power required for a BFR propellant load would be just over 12 gigawatts. However, the processing plant design to support a BFR is still incomplete.
5. Propellant Densification: In his presentation introducing the BFR, Elon Musk has indicated that this reusable rocket will use densified liquid oxygen and methane.12 Densification occurs with supercooling the cryogenic liquid propellant held just below the normal boiling point (NBP). The result is that a rocket vehicle’s propellant tank can carry more fuel in the same volume tank while lowering the vapor pressure and thus the tank operating pressure.
The smaller tank volume and thinner tank wall afforded by propellant densification results in an overall smaller and lighter vehicle with increased payload capability. So, by densifying both propellants, the BFR’s projected performance, efficiency, and profitability are all improved.
The power calculated above only brings the oxygen and methane gas to a NBP liquid state. This means that in order to provide the densified propellants, the processing plant will require an additional subsystem and more energy to further cool the propellants to densify them prior to BFR fueling.
In a 1997 Rockwell International patent,13 a cooling unit utilized a combination of heat exchanger and compressor. The compressor lowers the coolant fluid bath pressure, resulting in a low temperature boiling liquid that is subsequently used to cool the recirculating liquid. If we use the example from NASA’s X-33 liquid oxygen densification demonstrator facility, this processing consumed 500 KVA and had a reasonably efficient circuit power factor of 0.85.
In a 2000 NASA large-scale test in the X-33 program on the densification of cryogenic propellants through subcooling, it was shown that an average of 8.9 percent more propellant mass could be stored in a given unit volume.14 The design used a continuous liquid ixygen densification production process utilizing two shell and spiral coil heat exchangers in series.
The actual BFR propellant densification factor was unavailable at the time of publication and so only rough estimated factors are possible in this analysis. Further, densification test data and patents did not record energy consumption in the test data set or patent applications. At this point in the review process only a densification processing multiplying factor is used to account for energy needed. This densification factor is included as part of the final overhead multiplier added in the total power summation below.
6. Summing up the total power needed: In summary, this first order engineering process design exercise has reviewed a preliminary example Martian propellant production plant concept. Totaling the power requirements of subsystems and Martian water ore feed systems shows the projected power needs for a single BFR for the major parts of the propellant production process are just about 14.3 gigawatt-hours. However, there are also a number of additional overhead unaccounted power consuming parts of the plant, plus the densification power.
To account for these various additional overhead energy needs, an overhead multiplication factor has been added. This factor attempts to account for the energy for densification of the two propellants, plant pumps, operating control computers, and other electro-mechanical overhead power requirements, as well as any energy needed to keep the two propellants thermally managed during their storage. This power demand overhead factor also includes the densification power and so is estimated to the gross first order to add 15 percent to the total power budget. With this overhead factor, the total projected power needed to produce a single full load of propellant for a SpaceX BFR is in the neighborhood of 16 gigawatts of locally Martian-produced power.
The observant reader will note that this seemingly large massive total BFR propellant production power estimated need is not required instantaneously. Instead, it will be spread out over an extended production run. Assuming the BFR lands and launches when the Earth and Mars are best aligned for minimum energy transit, the single BFR propellant power demands create a power demand averaged over 26 months. In the next article we’ll look at where this power might come from and project a continuous production average power demand over an entire BFR propellant production run.
Endnotes
- Zubrin*, Robert, Frankie, Brian, and Kito, Tomoko, “Mars In-Situ Resource Utilization Based on the Reverse Water Gas Shift: Experiments and Mission Applications”, 1997 Pioneer Astronautics, AIAA 97-2767 or (Zubrin, Robert & Frankie, Brian & Kito, Tomoko. (1997). Mars in-situ resource utilization based on the reverse water gas shift - Experiments and mission applications. 10.2514/6.1997-2767).
- Ibid: reference 3. Robert M. Zubrin, Anthony C. Muscatello, and Mark Berggren.
- Bock, E.H., and Fisher, J.G., ”In-Space Propellant Processing Using Water Delivered as Shuttle Contingency Payload,” AIAA Paper 78-941, July 1978.
- Strobridge, T.R., "Cryogenic Refrigerators-An Updated Survey," NBS Tech. Note 655, rune 1974
- Bock, E.H., and Fisher, J.G., ”In-Space Propellant Processing Using Water Delivered as Shuttle Contingency Payload,” AIAA Paper 78-941, July 1978.
- Kohout, L. L., “Cryogenic Reactant Storage for Lunar Base Regenerative Fuel Cells,” NASA TM-101980, presented at the International Conference on Space Power (IAF), June 1989.
- “Space Based Cryogenic Fuel Station,” Rocketdyne Division, Rockwell International, viewgraph presentation to NASA Lewis Research Center (Advanced Space Analysis Office), July 1987.
- Moran, M., Conceptual Study of on Orbit Production of Cryogenic Propellant by Water Electrolysis, AOAA-91-1844, 27ty Joint Propulsion Conference, June 24-26, 1991.
- Qiang W Yanzhong L and Jiang W 2004 Analysis of power cycle based on cold energy of liquefied. natural gas and low-grade heat source, App. Thermal Engineering 24 539–548.
- Gerasimov V E Kuz’menko I F Peredel’skii V A and Darbinyan R V 2004 Introduction of technologies and equipment for production, storage, transportation, and use of LNG, Chemical and petroleum engineering 40 31-35.
- The Natural Gas Industry. A Review of World Resources and Industrial Applications, Medici, M. Published by London: {1974}, (1974), ISBN 10: 0408705469 ISBN 13: 9780408705462.
- Elon Musk Introduction of the Big Falcon Rocket, presentation video.
- US Patent number 5,644,920, LIQUID PROPELLANT DENSIFICATION, 8 July 1997, Rockwell International Corp.
- Liquid Oxygen Propellant Densification Unit Ground Tested With a Large-Scale Flight-Weight Tank for the X-33 Reusable Launch Vehicle, 2002.